After welding stainless steel, you’ll often see the weld bead lose its metallic luster, turning into various shades of blue, black, or brown. This discoloration, known as heat tint, is caused by high-temperature oxidation. While heat tint is an oxide, its structure is typically loose and uneven. When chromium oxidizes to form this heat tint layer on the surface, the metal beneath often becomes depleted of chromium, significantly reducing its corrosion resistance. Furthermore, a potential difference can form between the heat-tinted layer and the underlying base metal. When an electrolyte (like water or moist air) is present, this can accelerate galvanic corrosion, compromising the stainless steel’s integrity.
Stainless steel isn’t a natural element; it’s an engineered metal, primarily iron alloyed with elements like chromium (Cr), nickel (Ni), carbon (C), manganese (Mn), molybdenum (Mo), and silicon (Si). Its remarkable “stainless” quality comes mainly from chromium, which must constitute at least 10.5% of its composition. Chromium forms a passive, protective oxide layer on the surface when exposed to oxygen, often described as Cr2O3.
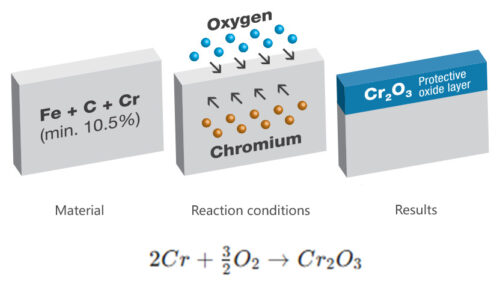
However, this vital chemical barrier is not invincible. It can be compromised by external factors such as iron contamination from carbon steel scratches, or by the high temperatures of welding, which cause the steel to appear blue or black. Both clearly indicate severe damage to the chromium protective layer. Without proper treatment, rust will soon cover the stainless steel.
This is where passivation becomes essential. It not only restores the protective chromium layer to prevent corrosion, but also effectively removes weld discolorations like heat tint, bringing back the metal’s original luster.
目录
切换Natural Weld Passivation vs. Artificial Weld Passivation
Natural Weld Passivation
In ideal conditions, stainless steel can self-passivate. This means its chromium spontaneously oxidizes to form a protective layer when the surface is clean and sufficient oxygen is present. This process is also called self-passivating. Unlike stainless steel, some “noble metals” like gold or platinum are inherently stable. They do not rely on forming a protective film to resist corrosion. Instead, their chemical inertness makes them highly unreactive and difficult to oxidize.
Advantages of Natural Passivation:
Natural passivation requires no additional processing, making it a low-cost option.
Disadvantages of Natural Passivation:
The natural passivation process is slow. For weld-affected areas, the naturally formed passive layer may be incomplete, uneven, or not dense enough to provide adequate corrosion protection. This is due to chromium depletion and the presence of contaminants. Weld areas may also show heat tint and “sugaring” (chromium carbides), which severely compromise corrosion resistance.
Artificial Weld Passivation
Artificial weld passivation accelerates and enhances the formation of the passive layer through chemical treatments. This effectively removes free iron and surface contaminants, forming a thicker, more uniform, and denser protective layer in a short period. This includes repairing heat-tinted areas. Heat tint is a serious contaminant that must be removed from the surface, not only for aesthetic reasons but also to prevent corrosion, as it indicates a compromised passive layer.
Disadvantages of Artificial Passivation:
Artificial passivation requires the use of chemicals, which can raise safety and environmental concerns.
Types of Artificial Passivation
Artificial passivation primarily involves two main categories of processes:
1. Traditional Chemical Passivation: This method uses specialized acid solutions to chemically dissolve contaminants like free iron and weld oxides from the surface. Once the surface is clean, the oxidizing agents in the solution help to rapidly form a new, stable chromium oxide passive layer. This process is effective for various stainless steel grades and can be applied in two primary ways:
Immersion: Smaller or complex parts are fully submerged in a bath of the passivation solution. This ensures complete and uniform coverage.
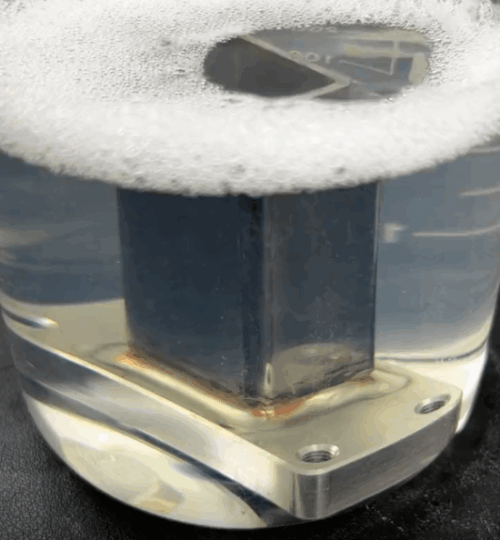
Paste/Gel Application: For larger structures or specific weld areas, the passivation solution is applied as a paste or gel directly to the surface. This allows for localized treatment without requiring a large immersion tank.

2. Electrochemical Passivation: Also known as electrocleaning or electropolishing, this process uses an electric current in conjunction with an electrolyte solution. The stainless steel part acts as an anode, and as current passes through, it removes surface imperfections, contaminants, and the compromised oxide layer. This method is highly effective at dissolving heat tint and promoting a highly uniform, clean, and chromium-rich surface, leading to an excellent passive layer. It provides precise control over the removal process and can result in a brighter, more aesthetically pleasing finish.

Regardless of the method chosen, it’s crucial to follow the specific process guidelines rigorously to ensure safety and effectiveness.
